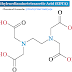
The proton-pump inhibitor pantoprazole inhibit gastric acid by blocking the H+ /K+ -adenosine triphosphatase enzyme system (the proton pump) of the gastric parietalcell. It is used for short-term treatment of erosion and ulceration of the esophagus. The pantoprazole oral dosage forms are supplied in enteric-coated tablets. Different analytical methods are reported in the literature for the assay of pantoprazole in dosage forms and in biological fluids including spectrophotometry, TLC,HPTLC Pantoprazole sodium is chemically Sodium 5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2- pyridinyl) methyl] sulfinyl]-1H-benzimidazole sesquihydrate. It has an empirical formula of C16H15F2N3O4S and molecular weight of 383.37.
Experimental
A High Performance Liquid Chromatograph system, with LC solutions data handling system (Shimadzu-LC2010) with an auto sampler was used for the analysis. The datawas recorded using LC 2010 solutions software. The purity determination performed on a stainless steel column 150 mm long, 4.6 mm internal diameter filled with Octadecyl silane chemically bonded to porous silica particles of 5µm diameter (Inertsil C18, 5µ , 150 mm x 4.6 mm, make: Shimadzu ltd, Japan) with the mobile phase containing acetonitrile and phosphate buffer in the ratio of 70:30 (v/v pH 7.0) at ambient temperature. Flow rate was kept at 0.8 ml/min and the elution was monitored at 260 nm
Materials and Chemicals
Pantoprazole sodium working standard, For the estimation of Pantprazole sodium in bulk and commercial formulations of pantoprazole sodium, 20 tablets were obtained from retail pharmacies. Each tablet was labeled contain 20 mg of pantoprazole sodium and had an expiry of not less than 365 days at the time of study. HPLC grade Sodium dihydrogen phosphate (NaH2PO4) .Disodium hydrogen phosphate (Na2HPO4), Acetonitrile, High pure water was prepared by using Millipore Milli Q plus purification system.
Preparation of mobile phase:
Mobile phase was prepared by mixing 700 ml of acetonitrile with 300 ml of phosphate buffer and its pH adjusted to 7.0. The mobile phase was sonicated for 15min and then it was filtered through a 0.45 µ membrane filter paper.
Preparation of stock and standard solutions:
Accurately weighed 25 mg of test sample into a clean dry 50 ml volumetric flask, dissolve and dilute to the mark with mobile phase. Mark this solution as sample solution. This solution contains 0.5 mg/ml of sample. Qualified working standard of Pnatoprazole sodium is used to carry out validation exercise. The potency of working standard is 99.75 %. With the optimized chromatographic conditions, a steady baseline was recorded, the standard solution was injected and the chromatogram was recorded. This procedure was repeated for the sample solution.
The method was validated for the parameters like specificity, range and linearity, limit of detection (LOD), limit of quantitation (LOQ), accuracy, and precision. In addition, system suitability parameters were also calculated. To demonstrate specificity in the presence of excipients used in formulation, pantoprazole sodium was spiked (at approximately 25 µg/ml) in drug product, chromatogram was observed and compared with that of raw material. To evaluate the linearity, the LOD and LOQ of the method in reference drug ,different serial dilutions (0.0980, 0.190, 0.80, 1.50, 3.10, 6.30, 12.50 and 25 µg/ml) were prepared from the standard stock solutions in 25 ml volumetric flasks and volume made up with diluent which is mixture of 70:30 acetonitrile & methanol. The samples were injected (10 µl) and signals from the samples were recorded at 2.02 minute which were compared with those of blank. LOD and LOQ values were calculated as signal-to-noise ratio of 3:1 and 10:1 respectively. To determine accuracy of the method, working standard of pantoprazole sodium was prepared in triplicate at three concentration levels (10, 20 and 25 µg/ml) and analyzed. Repeatability of the method was checked by analyzing six replicate samples of pantoprazole sodium (at the 100% concentration level) and calculating relative standard deviation (%RSD).
To determine intermediate precision, standard solutions of pantoprazole sodium at eight concentration levels were analyzed three times within the same day (intra-day variation) and three other days (inter-day variation).
Assay in formulations
In case of marketed formulations, five accurately weighed tablets were crushed to a fine powder and an amount equivalent to 10 mg of pantoprazole sodium was added into different 100 ml volumetric flasks and volume was made up with acetonitrile and methanol mixture. The samples were filtered through a 0.45-µm-membrane filter; different serial dilutions (3.10, 6.20, 12.40,25µg/ml) were made from this solution in 25 ml volumetric flask and were injected for HPLC analysis.
Results and discussion
For validation of analytical methods, the guidelines of the International Conference on the Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Human Use [ICH 1996] and [USP 2002] have recommended the accomplishment of accuracy tests, precision, specificity, linearity of the method
System suitability
The HPLC system was equilibrated with the initial mobile phase composition, followed by 10 injections of the same standard. These 10 consecutive injections were used to evaluate the system suitability on each day of method validation.
The system suitability parameters including capacity factor >2, resolution>3 and asymmetric factor<2. All parameters were satisfactory with good specificity for the stability assessment of pantoprazole sodium. Theoretical plates of the column were >3000.
Accuracy
The accuracy of an analytical method is the closeness of test results obtained by that method to true value. In case of the assay of a drug in a formulated product, accuracy may be determined by application of the analytical method to synthetic mixtures of the drug product components to which known amount of analyte has been added within the range of method. If it is not possible to obtain samples of all drug product components, it may be acceptable to add known quantities of the analyte to the drug product (i.e.,”to spike”) (USP 2004). In our studies, the later technique was adopted and pantoprazole was spiked in drug product. The result of accuracy given in (Table-1) revealed that the method was found accurate for all above purposes.
Precision is the degree of reproducibility or repeatability of the analytical method under normal operating conditions (USP 2004). The method passed the test for repeatability as determined by %RSD of the area of the peaks of six replicate injections at 100% test concentration. The results of intra-and inter-day variation are shown in (Table 2).
Limits of Detection and Quantitation
The detection limit (LOD) is the lowest amount of an analyte in a sample that can be detected, but not necessarily quantitated, under the stated experimental conditions. It may be expressed as a concentration that gives a signal-to-noise ratio of 2:1 or 3:1 (USP 2004, ICH Q2B guidelines, 1996 1997, FDA, Guidance for Industry 2000)13, 14. The lower limit of detection for rabeprazole is 2.40ng/ml in reference material and formulation and 1.70ng/ml serum. Limit of Quantitation (LOQ) is the lowest amount analyte in a sample that can be determined with acceptable precision and accuracy under the stated experimental conditions. A signal-to-noise ratio of 10:1 can be taken as LOQ of the method (USP 2004). The LOQ values were found to be 8.15ng/ml for raw material, formulations and 5.70ng/ml for serum.
Specificity
Specificity is the ability to assess unequivocally the analyte in the presence of components that may be expected to be present in the sample matrix (USP 2004). For demonstrating the specificity of the method for drug formulation the drug was spiked and the representative chromatogram (Figure-1). The excipiants used in different formulation products did not interfere with the drug peak and thus, the method is specific for pantoprazole. To further confirm the specificity of the method, UV scans of spiked drug were taken in the range 200-400nm and no significant change was found by comparing the absorbance of pure drug and spiked drug at the analytical wavelength of drug.








.JPG)











![Difference between Stability[Shelf life] Specification and Release Specification](https://4.bp.blogspot.com/-O3EpVMWcoKw/WxY6-6I4--I/AAAAAAAAB2s/KzC0FqUQtkMdw7VzT6oOR_8vbZO6EJc-ACK4BGAYYCw/w680/nth.png)

0 Comments